
By Robert W. George and Kael K. Bowling
Deadlines are fast approaching for food manufacturers to eliminate Partially Hydrogenated Oils (PHOs) from their products. In 2015, the Food and Drug Administration determined that PHOs were no longer Generally Recognized as Safe (GRAS) and established Monday, June 18, 2018 as the deadline for removal of PHOs from food product formulations.
The compliance deadline remains intact for most foods with a few exceptions. As a result of a petition by the Grocery Manufacturers Association, the agency recently extended the compliance date to Wednesday, June 19, 2019 for a limited number of categories of PHO food manufacturing uses and will allow additional time after PHO use in manufacturing for food products to clear the market through normal sale and distribution cycles.
The following chart summarizes the deadlines that food manufacturers, distributors and sellers need to be aware of:
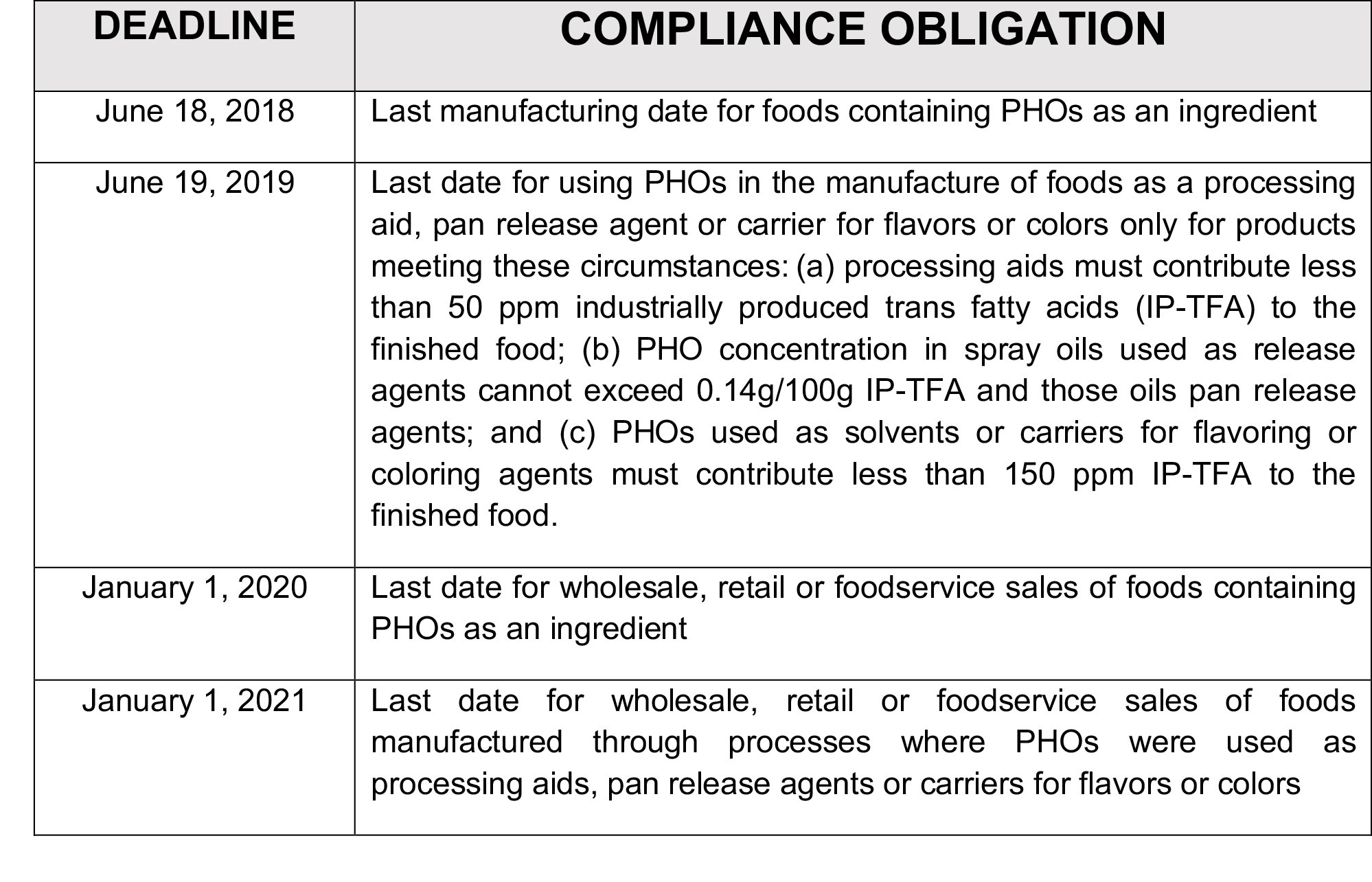
Businesses that manufacture or sell foods need to review the ingredients and manufacturing processes for the products they sell and take steps now to ensure compliance with legal requirements. FDA has given the food industry three years to reformulate products and has granted additional time for a limited number of PHO uses and for products produced with PHOs to clear the market, but the deadlines for compliance are fast approaching. The food industry could begin to see FDA enforcement actions related to PHO use beginning in June of this year.
Determining compliance with FDA’s PHO ban presents a series of legally and technically complex questions. Our firm would be pleased to assist you in developing and implementing a plan to ensure that your company meets its legal obligations related to PHO use in foods.
The information provided above is created the Attorneys Robert W. George and Kael K. Bowling of Friday, Eldredge & Clark, LLP. Robert’s focus consists of a multifaceted business litigation practice in both federal and state courts and includes defending clients against environmental and toxic tort claims, as well as commercial disputes or involving fraud, theft or unfair competition. Kael is an associate in the firm’s Commercial Litigation and Regulation Practice Group. His practice includes serving as litigation counsel to financial institutions, insurance companies, and other business entities in connection with a broad range of subject matters.
This is not a substitute for legal advice and should be considered for general guidance only. For more information or if you have further questions, please contact one of our attorneys.